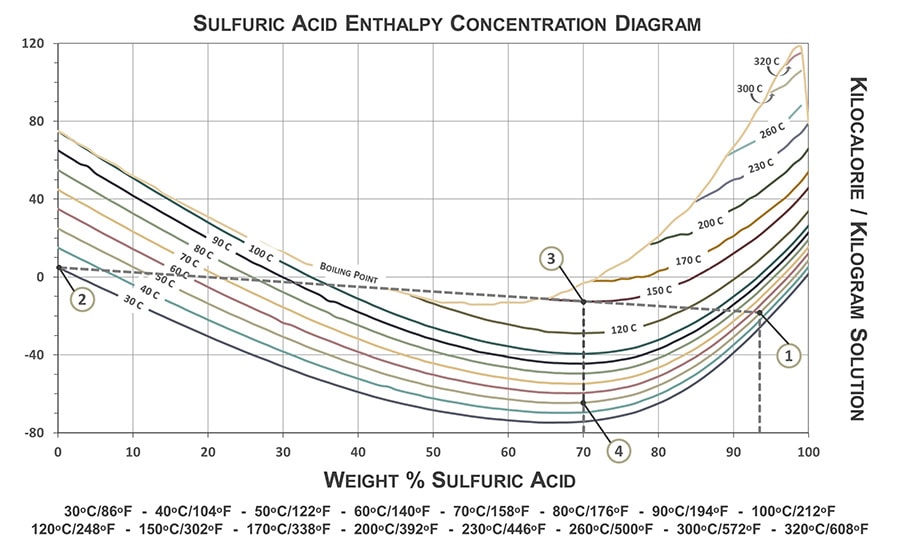
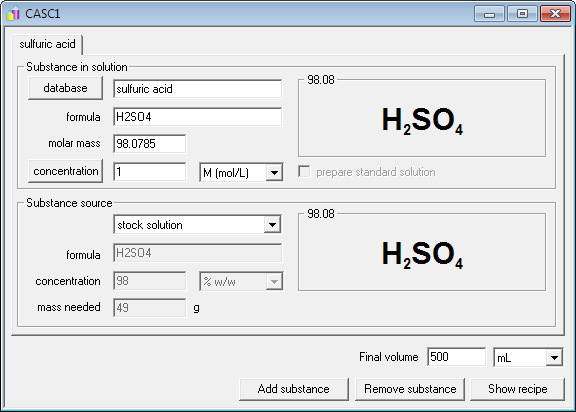
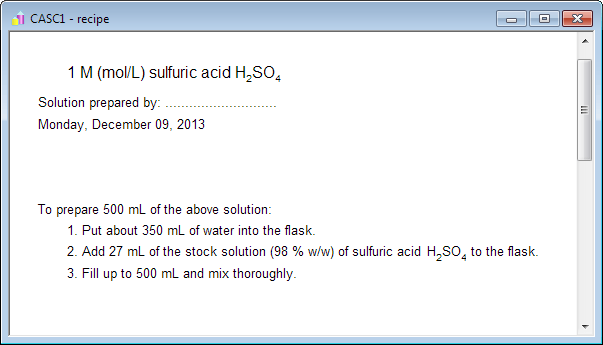
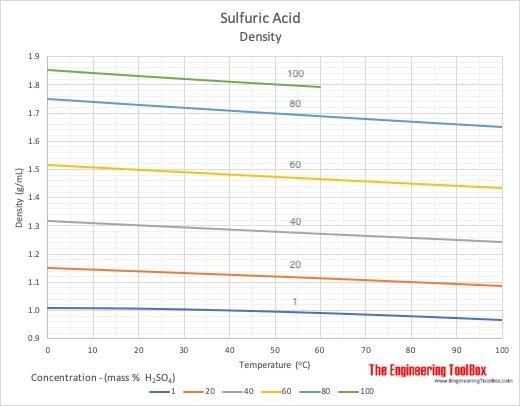
How much heat does 240 millilitres sulfuric acid make when 240 millilitres of water is added? - Quora

Question Video: Calculating the Volume of Sulfuric Acid That Completely Neutralizes a Given Volume and Concentration of Sodium Hydroxide | Nagwa
![SOLVED: A solution of sulfuric acid has a pH of 2.55. Calculate the following: a) [H+] (3 marks) b) The concentration of the sulfuric acid solution *Assume the ionization is complete and SOLVED: A solution of sulfuric acid has a pH of 2.55. Calculate the following: a) [H+] (3 marks) b) The concentration of the sulfuric acid solution *Assume the ionization is complete and](https://cdn.numerade.com/ask_previews/5338532b-72ac-4610-a96e-d01f0328e5e1_large.jpg)
SOLVED: A solution of sulfuric acid has a pH of 2.55. Calculate the following: a) [H+] (3 marks) b) The concentration of the sulfuric acid solution *Assume the ionization is complete and

Molarity by Dilution Diluting Acids How to Calculate Acids in concentrated form are diluted to the desired concentration using water. Moles of acid before. - ppt download




:max_bytes(150000):strip_icc()/how-to-calculate-normality-609580final2-0d5efa5a961f4fa0a7efc780921faee1.png)